➧ Spinal (intrathecal) anesthesia is one of the most reliable regional block methods: the needle insertion technique is relatively straightforward, with cerebrospinal fluid (CSF) providing both a clear endpoint of successful needle placement and a medium for carriage of local anesthetic (LA) within subarachnoid space. However, the possibility of failure has long been recognized, with an incidence of less than 1% in experienced hands.
➧ Literally, the word failure implies that spinal anesthesia was attempted, but no block resulted or a block results, but is inadequate for the proposed surgery.
➧ Such inadequate block may be related to the three components of the block: the extent, the quality, or the duration of local anesthetic action, often with more than one of these being inadequate.
➧ The intrathecal injection can go astray within each of the five phases of an individual spinal anesthetic, leading to blocking failure, these being, in sequence:
1-Lumbar puncture
2-Solution injection
3-Spreading of the drug through CSF
4-Drug action on the spinal nerve roots and cord
5-Subsequent patient management
Causes and prevention of failure:
1-Unsuccessful Lumbar Puncture (Dry Tap):
-Inability to obtain CSF ‘Dry Tap’, is the only cause of failure which is immediately obvious.
Causes:
a) Incorrect needle insertion or Poor patient positioning:
Prevention:
➧ A calm, relaxed patient is more likely to assume and maintain the correct position, so:
-Explanation (before and during the procedure) and gentle, unhurried patient handling are vital.
-Premedication with light anxiolytic for relaxing the patient.
-Local anesthetic infiltration at the puncture site is effective without obscuring the landmarks.
-Systemic analgesia (IV or inhalation) helps in achieving the correct position for patients in pain (e.g. from a fractured hip).
b) Anatomical abnormalities of the spine:
-Kyphosis, scoliosis, calcification of ligaments, consequences of osteoporosis, obesity, and patient anxiety, make both positioning the patient and needle insertion more difficult, especially in the elderly.
Prevention:
-Good clinical training is the key to success.
-Adherence to the basic rules of positioning, needle insertion, and use of adjuncts.
-Lateral or paramedian approach, especially if the mid-line ligaments are heavily calcified.
-Ultrasound guidance: a pre-procedure scan can be useful in patients with anatomical abnormality to identify the midline and level of injection and to assess the depth of dura from the skin.
c) Equipment-related factors: A blocked needle lumen:
Prevention:
-Both needle and stylet must be checked for correct fitness before use.
-The needle should not be advanced without the stylet in place, because tissue or blood clots can easily obstruct the fine bore needles used now.
-Prompt needle withdrawal and 'flush test' to assure patency.
2-Pseudo-successful Lumbar Puncture:
-The appearance of clear fluid at the needle hub is usually the final confirmation that the subarachnoid space has been entered.
a) Epidural 'Top-up' dose:
-Rarely, the clear fluid is not CSF, but LA injected as an epidural ‘top-up’ dose or spreading from the lumbar plexus.
Prevention:
-Unfortunately, a positive test for glucose in the fluid does not confirm that this fluid is definitely CSF because extracellular fluid constituents diffuse rapidly into fluids injected into the epidural space.
b) Congenital arachnoid cyst:
-Another, rarer cause is a congenital arachnoid cyst (Tarlov cyst), which are meningeal dilatations of the posterior spinal nerve root, present in 4.5-9% of the population.
3-Solution Injection Errors:
1. Dose selection:
-The dose injected, within the normal range, has only a small effect on the height of a spinal block but is important in determining the quality and duration of the block.
➧ The dose chosen will depend on:
-The specific LA used
-The baricity of LA solution
-The patient’s subsequent posture
-The type of block intended
-The extent and duration of planned surgery
Causes:
➧ Some anesthetists use lower doses than is traditional, in attempts to either:
-Minimize hypotension, by producing a unilateral block.
-Decrease block duration which speeds postoperative mobilization and decreases the need for bladder catheterization.
➧ Such lower doses will increase the margin for error and exaggerate the consequences of other problems such as:
-Loss of injectate and so risk an inadequate block.
-The ‘dead space’ of the needle and hub will contain a significant proportion of what is a small volume to start with.
2. Loss of injectate:
Causes:
-Leakage of LA solution through the Luer connection between syringe and needle.
-Leakage through a defect at the junction of needle hub and shaft.
➧ Given the small volumes involved, the loss of a few drops can cause a significant decrease in the mass of the drug reaching the CSF, and thus in its effectiveness.
Prevention:
-Insert the syringe containing the injectate firmly into the hub of the needle, and check that no leakage occurs.
3. Misplaced injection:
Causes:
➧ Anterior or posterior displacement of the needle tip from subarachnoid to epidural space:
a) During connection of the syringe to the needle, where deposition of a spinal dose of LA will have little or no effect.
b) During fluid aspiration for confirmation that the needle tip is still in the correct space, may displace the tip unless performed carefully, as may the force of the injection of the syringe contents.
Prevention:
-The dorsum of one hand should be anchored firmly against the patient’s back and the fingers are used to immobilize the needle, while the other hand is used to manipulate the syringe.
-After attachment of the syringe, aspirate 0.5-1 ml to confirm the free flow of CSF, and at the end of spinal injection, aspirate 0.5-1 ml, to confirm that the needle tip is still in the subarachnoid space, the aspirated volume is re-injected before the needle is withdrawn. Some anesthetists advocate that this is done halfway through as well,
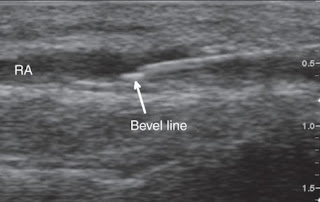
c) Tip displacement is an important issue with the ‘Pencil point’ needles, as the opening at the end of these needles is proximal to the tip, so only a minor degree of ‘backward’ movement during syringe attachment may result in epidural injection.
-Also, the opening of these needles may ‘straddle’ the dura so that some solution reaches the CSF, and some of the epidural space.
-This may be exaggerated by the dura acting as a ‘flap’ valve across the needle opening. Initially, CSF pressure pushes the dura outwards so that aspiration is successful, but subsequent injection pushes the dura forward and the solution is misplaced.
-A variant is that the needle tip penetrates the dura, but it is the arachnoid mater that acts as the flap valve so that accidental subdural injection results.
Prevention:
-Rotation of the needle through 360 degrees after the initial appearance of CSF, and before check aspiration, as the rotation reduces the risk of the membrane edges catching on the opening.
4. Inadequate intrathecal spread:
➧ Factors affecting the intrathecal spread of a local anesthetic solution:
-Anatomy of the vertebral canal.
-Solution physical characteristics.
-Gravity.
a) Anatomical abnormality:
-Abnormalities of the curves of the vertebral column as kyphosis or scoliosis, may interfere with the solution spread.
Examination of the patient should reveal whether this might occur, but it is not possible to predict whether the effect will be excessive spread or failure.
-A rare possibility, is that the ligaments supporting the spinal cord within the theca, form complete septae which act as longitudinal or transverse barriers to LA spread. This can result in a block that is entirely unilateral or limited cephalad spread.
-Spinal stenosis or other pathological lesions can limit spread, effectiveness, or both.
-Previous spinal surgery or intrathecal chemotherapy may result in adhesions that interfere with LA spread.
-Increased CSF volume in the lumbar theca can cause restricted cephalad spread of intrathecal injection.
-A variation of this factor is dural ectasia, which is a pathological enlargement of the dura seen in most patients with Marfan’s syndrome and in some other connective tissue disorders.
b) Solution density (baricity):
-Isobaric solutions, with a density within the normal range of CSF, will block the lower limbs with little risk of thoracic nerve block and thus less hypotension.
-Plain solutions of bupivacaine, although referred to as isobaric, are actually of lower density to be hypobaric at body temperature (37ºC). They have a less predictable spread than that of a truly isobaric preparation, and the block may be not higher than the second lumbar dermatome with slow onset.
-Hyperbaric solutions, with a density greater than that of CSF, move under the combined influence of gravity and the curves of the vertebral canal. If the patient is placed supine after the injection of a hyperbaric preparation at the mid-lumbar level, the solution will spread ‘down’ the slope under the effect of gravity to pool at the ‘lowest’ point of the thoracic curve, so exposing all nerve roots up to that level to an effective concentration of LA. (Figure 1)
-However, if a lumbar puncture is performed at the fourth lumbar or the lumbosacral interspace, LA may be ‘trapped’ below the lumbar curve, especially if the patient is in the sitting position during injection and maintained in that position for a period thereafter. This results in a block that is restricted to the sacral segments.
-Chemical reaction can generate an obvious precipitate, or lower the pH of the LA solution which will decrease the concentration of the unionized fraction which diffuses into nerve tissue resulting in a decreased effect.
-Local anesthetics are compatible with most opioids, but the situation is less definitive with other adjuvants such as clonidine, midazolam, ketamine, and other substances.
-The stability is unknown when mixing three or more substances together for intrathecal use.
-The incidence of failure is greater after the addition of a vasoconstrictor solution.
c) Inactive LA solution:
-Ester-type LAs, are chemically labile so that heat sterilization and prolonged storage, particularly an aqueous solution, can make them ineffective because of hydrolysis, and hence they need very careful handling.
-Amide-type LAs (e.g. lidocaine, bupivacaine, etc.) are more stable and can be heat sterilized and stored for several years without loss of potency.
d) Local anesthetic resistance:
-Very rarely, failed spinal block has been attributed to physiological resistance to the actions of LA drugs.
-This problem is due to mutation of sodium channels (channelopathy) which is associated with significant neurological diseases such as; intractable epilepsy and chronic pain, however, this does not exist in asymptomatic individuals.
-A history of repeated failure of dental or other LA techniques is accompanied by speculation that the problem is due to sodium channel mutation that renders the drugs ineffective.
4-Failure of Subsequent management:
➧ Not all of a patient’s claims of discomfort, or pain, during spinal anesthesia, are due to inadequate block.
Causes:
-Lying awake during surgery is not a pleasant experience for most patients, and anxiety alone can cause patient discomfort.
-Furthermore, operating tables are designed for surgical access, not patient comfort; and intra-abdominal stimuli can result in afferent impulses in unblocked parasympathetic nerve fibers causing unpleasant sensations.
Prevention:
-Good preoperative patient counseling followed by a supportive approach from the anesthetist during the operation is important in avoiding such problems.
-Judicious, and proactive use of systemic analgesic drugs.
-Sufficient sedation to produce drowsiness, or even sleep (with appropriate monitoring), is indicated except in obstetric situations, where small doses may be useful.
-Distraction techniques such as listening to music.
5-Testing the block:
-It is mandatory to test the level of the block before surgery commences
-Most patients will have some anxiety about the effectiveness of the injection, and this will be increased if testing is started too soon.
-Conventional practice is to check motor block by testing the ability to lift the legs, followed by testing of sensory block to stimuli such as light touch, cold, or pin-prick.
-It is advisable to start testing in the lower segments, where onset will be fastest, and work upwards. Proving early that there is some effect encourages patient confidence; testing too soon does the opposite.
-Establishing that the level of block is appropriate for the projected surgery is often taken to demonstrate that the quality of block is adequate also.
-A covert pinch of the site of the proposed surgical incision may be a better indicator of skin analgesia and can be reassuring if the block has been slow in onset. Asking the surgeon to do the same with toothed surgical forceps after distracting the patient with conversation.
6-Catheter and Combined Techniques:
-The majority of spinal anesthetics involve a single shot of LAs.
-To take advantage of the rapid onset and profound block of spinal anesthesia, both continuous and combined spinal-epidural techniques have been introduced to increase flexibility.
-If the catheters are correctly placed, problems of inadequate spread, quality, and duration of effect can be dealt with.
-However, insertion of an intrathecal catheter can be difficult to achieve in some patients and can result in the misdirection of the LA solution, with the risk of neurotoxicity.