Anesthetic Management of Down's Syndrome
➧ This well-known syndrome, with characteristic morphological features and mental retardation, results from the chromosomal abnormality, trisomy 21.
➧ Anesthetic risk is increased in these children. Indeed, the mortality is increased at any stage of life, but improved medical and nursing care means that many more individuals are surviving into adulthood and may present for surgery.
➧ Between 60 and 70% of patients now survive beyond 10 years of age.
Preoperative abnormalities:
1. Cardiac abnormalities: occur in 50–60% of patients and are usually responsible for the initial mortality in infancy. The commonest lesions are septal defects, Fallot’s tetralogy, and patent ductus arteriosus. In adults, there is an increased risk of mitral valve prolapse and mitral and aortic valve regurgitation.
2. Immune system defect: results in an increased incidence of infection. Granulocyte abnormalities decreased adrenal responses, and defects in cell-mediated immunity have all been identified. There is an increased incidence of lymphomas and leukemias.
3. Skeletal abnormalities: atlantoaxial instability was recognized as being a problem, at a time when these children were encouraged to participate in gymnastics. Down’s children may have C1–C2 articulation abnormalities, subluxation, and odontoid peg abnormalities. It may occur in association with either medical procedures or physical activity. The cause of instability may be due to: poor muscle tone, ligamentous laxity, and abnormal development of the odontoid peg.
4. Biochemical abnormalities: involve the metabolism of serotonin, catecholamines, and amino acids.
5. Thyroid hypofunction: is common in both adults and children, although hyperthyroidism can sometimes occur. A child with Down’s syndrome had a thyrotoxic crisis that mimicked malignant hyperthermia.
6. Sleep-induced ventilatory dysfunction: has been reported.
7. Institutionalized Down’s patients have an increased incidence of hepatitis B antigen.
8. Autonomic dysfunction: in particular increased sympathetic function and decreased vagal activity, result from brainstem abnormalities.
Anesthetic problems:
1. Cervical spine abnormalities: increase the risk of dislocation of certain cervical vertebrae on intubation, or when the patient is paralyzed with muscle relaxants with risk of atlantoaxial subluxation and spinal cord compression. Cervical spine screening has to be carried out, and precautions taken during intubation.
2. The larynx is often underdeveloped and smaller tracheal tube size is required than would be anticipated for the age of the patient. The adult larynx may only accept a size 6-mm tube.
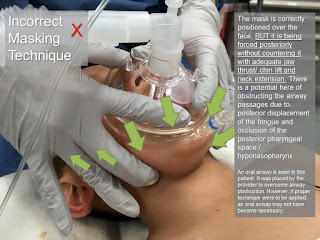
3. Airway and intubation difficulties sometimes occur, from a combination of anatomical features. These include a large tongue, a small mandible and maxilla, a narrow nasopharynx, and irregular teeth. Even in the absence of teeth, intubation is made more difficult by excessive pharyngeal tissue.
4. Postoperative stridor after prolonged nasal intubation. Congenital subglottic stenosis occurs occasionally.
5. Obstructive sleep apnea is common in Down’s syndrome. Compared with normal children they had an increased incidence of stridor and chest wall recession, lower baseline oxygen saturations, and a greater number of episodes of desaturation to 90% or less. Chronic episodes of hypoxia and hypercarbia may lead to pulmonary hypertension and congestive heart failure. Airway patency depends upon both the anatomical structure of the upper respiratory tract and the normal functioning of the pharyngeal muscles. Abnormalities of either or both may occur.
6. Upper airway obstruction, because it has multiple causes, is not necessarily resolved with surgical treatment. Young patients with more severe symptoms often had multiple sites of obstruction and a high incidence of cardiac disease.
7. Problems of the associated cardiac disease, which in later life may lead to pulmonary hypertension.
8. A high incidence of atelectasis and pulmonary edema after surgery for congenital heart disease. Those with Down’s syndrome and ventricular septal defects were predisposed to pulmonary vascular obstruction.
9. Posterior arthrodesis of the upper cervical spine carries a high complication rate. Problems included infection and wound dehiscence, instability at a lower level, neurological sequelae, and postoperative death.
Management:
1. Lateral cervical X-rays are required, in full flexion and extension positions, to detect atlantoaxial instability. This may show as an increase in the distance between the posterior surface of the anterior arch of the atlas, and the anterior surface of the odontoid process. Patients with an atlanto-odontoid interval of 4.5–6.0 mm were asymptomatic, but those in whom the distance exceeded 7 mm had neurological signs. If instability is present, great care should be taken to immobilize the neck during intubation and muscle relaxation. These changes do not appear to progress with time.
2. If a significant cardiac disease is present, management must be appropriate to the lesion, and endocarditis prophylaxis is given as recommended.
3. A tracheal tube should be used that is 1–2 sizes smaller than would be expected from the patient’s age.
4. If prolonged nasotracheal intubation is required, steroids should be given before extubation. The child should receive humidification and be observed carefully for signs of stridor.
5. Close observation is required in the perioperative period, to detect episodes of obstructive apnea. A pulse oximeter is useful.
6. Loss of locomotor skills or disturbances of gait after surgery or acute trauma should alert staff to the possibility of subluxation and cord compression. In the event of this, an urgent neurological opinion should be sought. However, in the absence of neurological signs, non-operative management has been advised, because of the high complication rate after surgery.
7. In patients with adenotonsillar hypertrophy, surgery may improve obstruction. However, close monitoring and oxygen therapy are important for the first postoperative night.