Categories
- Airway
- Anesthesia machine
- Anesthetic drugs
- CNS diseases
- Complications in anesthesia
- Congenital cardiovascular diseases
- Endocine Emergencies
- Fluid & Electrolyte disturbances
- GIT diseases
- ICU
- Inborn errors of metabolism
- Infections
- Monitoring
- Neuropsychiatric diseases
- Nutritional
- Obstetric anesthesia
- Ophthalmic anesthesia
- Otorhinolaryngology
- Pediatric anesthesia
- Plastic
- Regional anesthesia
- Renal diseases
- Research
- Respiratory diseases
- Skin & Musculoskeletal diseases
- Thoracic surgery
Failed Epidural Block
By Author April 01, 2021
Failed Epidural Block
Introduction:
➧ Failure of epidural anesthesia and analgesia occurs in up to 30% of clinical practices.
➧ Some technical factors can help to increase the primary and secondary success rates.
A) Technical factors influencing block success:
1-Patient Position:
➧ Patient positioning potentially affects needle placement by changing the relationship between osseous and soft tissues.
a) Sitting Position
➧ Results in shorter insertion times and higher accuracy at the first attempt than in the lateral position.
➧ Causes more vagal reflexes than lateral position.
➧ This leads to epidural venous plexus distension, which may theoretically increase the risk of vascular puncture, especially in parturients.
b) Lateral Position
➧ Increases the distance from the skin to the epidural space.
➧ Results in more technical difficulties compared with the sitting position.
2-Puncture Site:
➧ Inaccurate dermatomal block-level or anatomical landmarks of neuraxial puncture are not suitable for the type of surgery.
3-Approach:
a) Midline Approach
➧ Results in a higher incidence of paresthesia than the paramedian approach.
➧ Results in a bloody puncture in non-pregnant adults than paramedian approach.
➧ The ligamentum flavum is not continuous in all patients, and the presence of midline gaps may make the loss of resistance (LoR) to needle advancement and injection of air/saline less perceptible when the midline approach is used.
b) Paramedian Approach
➧ Results in faster catheter insertion times, and less dependent upon spine flexion.
➧ Paramedian catheters cause less epidural tenting and pass cephalad more reliably than midline catheters.
4-Identification of the Epidural Space:
➧ Correct placement obviously requires correct identification of the epidural space. The LoR using saline has become the most widely used method, while LoR to air and the hanging drop technique is less widely used with no difference in the success rate or adverse events, other than a 1.5% reduction in post-dural puncture headache [1] when using saline.
➧ In obstetric epidurals, using saline for LoR results in fewer attempts than using air, but with comparable final success rates.
➧ The use of the ‘preferred technique’ (i.e. the technique used by anesthetists 70% of the time) results in significantly fewer attempts, a lower incidence of paresthesia, and fewer dural punctures, irrespective of whether saline or air is used for LoR.
➧ The hanging drop technique depends on negative pressure within the epidural space and is useful only in the sitting position.
➧ Identification of the epidural space was reported at 2 mm deeper for the hanging drop when compared with LoR, possibly indicating an increased risk of dural puncture.
➧ Ultrasound pre-assessment of lumbar epidural space depth has been shown to correlate well with actual puncture depth in obese parturients.
➧ The use of ultrasound led to less bony contact, a shorter time to block success, and decreased supplemental opioid requirements.
5-Epidural Catheter Location:
➧ Epidural catheters may primarily be placed incorrectly, or become dislodged during operation.
➧ Primary misplacement of epidural catheters in the paravertebral space, in the pleural cavity, or intravascularly.
➧ Transforaminal migration of the catheter tip and asymmetric spread during epidural analgesia.
➧ During normal patient movement, epidural catheters may be displaced by centimeters.
➧ Changes in epidural pressure and cerebrospinal fluid (CSF) oscillations can contribute to the displacement of epidural catheters.
➧ Midline fat pedicles may form a barrier to the spread of local anesthetics (LA).
6-Catheter Insertion and Fixation:
➧ The catheter should be inserted at least 4 cm into the epidural space.
➧ Suturing of the epidural catheter is associated with less migration, but at the cost of increased inflammation at the puncture site.
➧ Tunneling the epidural catheter for 5 cm is associated with less movement of the catheter and decreases catheter migration but it will not maintain the original position.
➧ Tunneling caudal epidural catheter in children reduces the risk of bacterial colonization to levels comparable to un tunneled lumbar catheters because tunneling places the catheter entry point above the diaper in babies and toddlers.
➧ For lumbar and epidural catheters, the advantages of tunneling are less obvious and the need to prevent dislodgement must be weighed against the increased incidence of erythema at the puncture site, potentially linked to increased risk of bacterial colonization.
➧ Catheter fixation devices are available which may significantly reduce migration percentage and reduce rates of analgesic failure.
7-Test Dose:
➧ A test dose is given with two main objectives of detecting intrathecal or intravascular catheter placement.
➧ A test dose of lidocaine (to detect intrathecal placement) and epinephrine (to detect intravascular placement) is recommended in patients without contraindications to epinephrine.
➧ Specific regimens to detect intravascular catheter position:
-Fixed epinephrine test dose for non-pregnant adult patients.
-Fentanyl test dose for parturients.
-Weight-adjusted epinephrine test dose for children.
➧ Patients sensitive to intravascular epinephrine (parturients, patients with cardiac or vascular disease) may experience undesirable side effects if the test is positive. However, this risk is outweighed by the systemic toxic effects of LA if the intravascular placement is not detected.
8-Equipments:
➧ The orifice of the catheter can lie laterally or anteriorly in the epidural space putting the LA more to one side and producing a unilateral block. In general, multi-orifice catheters are considered better than single-orifice catheters.
➧ Manufacturing errors, such as faulty markings on the epidural catheter, can lead to a wrong depth of placement.
➧ Debris in the catheter or disconnection can cause epidural failure.
➧ Obstruction of the epidural infusion system by an airlock, as little as 0.3–0.7 ml of air, in the bacterial filter.
➧ The knotting of the catheter internally or externally can cause obstruction.
➧ Removal of a presumed knotted catheter can be attempted after sensation has returned to monitor neurological symptoms during catheter removal. When radicular symptoms or pain occur during the removal of a catheter, this should be immediately stopped. It has been found that removal is easiest if the patient is in the same position as at insertion. Surgical removal of a broken catheter is not compulsory if the patient remains asymptomatic.
References:
1. Shaat AM, Abdalgaleil MM.
Is theophylline more effective than sumatriptan in the treatment of post-dural
puncture headache? A randomized clinical trial.
Egyptian Journal of Anaesthesia 2021; 37(1): 310-316.
https://doi.org/10.1080/11101849.2021.1949195.
Read more: ☛ Failed Spinal Anesthesia
Local Anesthetic Toxicity
By Author April 01, 2021
Local Anesthetic Toxicity
Clinical Manifestations & Management:
1-CNS manifestations:
➧ Early signs: circumoral numbness (Earliest sign), tongue paresthesia, dizziness
➧ CNS Excitation: (restlessness and agitation)
➧ CNS Depression: (slurred speech, drowsiness, unconsciousness)
➧ Muscle twitching, tonic-clonic seizures
➧ Respiratory arrest often follows
Management:
1-Stop LA injection or infusion
2-Call for help
3-Oxygenation
4-Hyperventilation (to decrease cerebral blood flow)
5-Anti-seizures: Benzodiazepines (diazepam 0.1-0.2 mg/kg), Thiopental (1-2 mg/kg)
2-Respiratory manifestations:
➧ Local anesthetics (LAs) depress hypoxic drive (ventilatory response to low PaO₂)
➧ Apnea can result from phrenic and intercostal nerve paralysis
Management:
➧ Respiratory support
3-CVS manifestations:
➧ In general, LAs depress myocardial automaticity (spontaneous phase IV depolarization) and reduce the refractory period causing: bradycardia, heart block (varying degrees), and hypotension which may lead to cardiac arrest.
➧ The R (+) isomer of bupivacaine rapidly blocks cardiac sodium channels and dissociates slowly.
➧ Major cardiovascular toxicity requires about 3× the concentration required to produce seizures.
➧ Ropivacaine is 70% less likely to cause severe cardiac arrhythmias than bupivacaine.
Management:
1-ACLS protocols
2-Vasopressors: Ephedrine, Norepinephrine, Epinephrine
3-Lipid emulsion infusion:
➧ Use of Lipid Emulsion:
-IV bolus of Intralipid 20% (1.5 ml/kg over 1 min) about 100 ml and start infusion of (0.25 ml/kg/min)
-If adequate circulation has not been restored: Repeat the bolus dose twice at 5 min intervals and increase the infusion rate to (0.5 ml/kg/min)
-Continue infusion until adequate circulation has been restored
-Measure LA & triglyceride levels
Patient-related factors to consider during large volume peripheral nerve block (PNB):
1-Age:
➧ Newborns have about half the adult concentration of α-acid glycoprotein (AAG) which binds free LAs.
➧ Persons over 70 years show increased sensitivity to LAs and decreased clearance.
Recommendation: Reduce LAs dose by 10-20%.
2-Renal dysfunction:
➧ There may be a change in clearance of LAs in uremic patients.
➧ Uremic patients show a rapid rise in LAs plasma levels probably secondary to a hyperdynamic circulation, but uremic patients have increased AAG levels.
Recommendation: Reduce LAs bolus and continuous dose by 10-20% in uremic patients.
3-Hepatic dysfunction:
➧ Clearance of LAs can be dramatically decreased but plasma concentrations remain close to normal secondary to increased volume of distribution (Vd). These patients also can have renal and cardiac dysfunction.
Recommendation: Initial bolus dose can be in the normal dose range but the continuous infusion dose should be reduced by 10-50%.
4-Heart Failure:
➧ Decreased blood flow to the liver and kidneys can cause a decrease in clearance
Recommendation: Repeat or continuous dosing of LAs should be reduced by 10-20%
5-Pregnancy:
➧ Progesterone may increase the sensitivity of nerve axons
➧ There is an enhanced risk of cardiotoxicity by bupivacaine and ropivacaine induced by progesterone. Increased cardiac output causes increased uptake of LAs
Recommendation: Avoid large volume PNB in 1st trimester and reduce doses in epidural and spinal anesthesia in pregnancy
6-Drug interaction:
➧ Amide LAs are cleared by the liver cytochrome P450 enzymes
➧ Propanolol, Cimetidine and Itraconazole can decrease bupivacaine clearance by 30-35%
➧ Ciprofloxacin and Fluvoxamine decrease the clearance of ropivacaine
Recommendation: Single bolus dose is of little concern but continuous infusion should be altered (10-20% decrease)
Bronchial Asthma
By Author January 01, 2021
Anesthetic Management of Pt. with Bronchial Asthma
Definition:
➧ It is an acute obstructive pulmonary disease. It is due to the hyperreactivity of the tracheobronchial tree, in which some exogenous or endogenous stimuli can produce reversible airway obstruction.
➧ Histamine and the leukotrienes are the most active chemical mediators, while acetylcholine contributes via a disturbance in autonomic balance by stimulation of M3 cholinergic receptors on bronchial smooth muscles causing bronchospasm.
Pathological changes: (Figure 1)
-Prolonged expiration
-Increased residual volume and functional residual capacity
-Increased work on breathing
-Reduced vital capacity, inspiratory capacity, and expiratory reserve volume
-Ventilation-perfusion inequalities
-Hypoxia
-Bronchospasm
-Mucus secretion
-Edema of the bronchial mucosa
-Epithelial desquamation
 |
| Figure 1: Pathological changes in Br. Asthma |
Anesthetic Management:
Preoperative:
a) Assessment:
1-History:
-Wheezing, dyspnea, cough, and sputum production.
2-Classification of Asthma:
-Mild Intermittent: Mild symptoms up to two days a week and up to two nights a month
-Mild Persistent: Symptoms more than twice a week, but no more than once in a single day
-Moderate Persistent: Symptoms once a day and more than one night a week
-Severe Persistent: Symptoms throughout the day on most days and frequently at night
3-Drug Therapy:
-β2-adrenoceptor agonists (albuterol, levalbuterol, salbutamol, terbutaline, salmeterol, formoterol)
-Xanthine derivatives (theophylline, aminophylline)
-Anticholinergics (Ipratropium bromide)
-Corticosteroids (beclometasone, budesonide, fluticasone, flunisolide, ciclesonide, mometasone).
-Mast cell stabilizers (sodium cromoglycate, nedocromil sodium)
-Leukotriene modifiers (montelukast, pranlukast, zafirlukast, zileuton)
-Allergy medications (allergy shots (immunotherapy), omalizumab)
4-Examination:
-Pulsus paradoxus
-Chest auscultation: The chest may be hyper resonant, with diminished breath sounds, prolonged expiration, and audible wheezes. In very severe cases the wheezes disappear
5-Investigations:
1. CBC:
-Polycythemia (if there is chronic hypoxemia)
-Eosinophilia: indicates the degree of airway inflammation
-Neutrophilia: bacterial infection
-Lymphocytosis: viral infection
2. Chest X-ray (CXR):
-Baseline for postoperative follow up
-May show an increase in bronchovascular markings and hyperinflation
-In the later stages, may show some degree of emphysema
-In an acute asthma attack, a CXR is essential to exclude pneumothorax
3. Arterial Blood Gases (ABG):
-Hypoxemia, Hypercarbia
4. Pulmonary Function Tests (PFT):
a) Tests indicate Severe Obstruction:
-Peak Expiratory Flow Rate (PEFR) of less than 120 l/min.
-Maximum Voluntary Ventilation (MVV) of less than 50% of predicted
b) Tests indicate the need for Postoperative Mechanical Ventilation (MV):
-FEV1 of less than 1 liter
-FEV1/VC ratio of less than 40% of predicted
-Increased PaCO₂ > 50 mmHg
c) Assessment of Severity of Asthma by PFT:
-Mild, Moderate, Severe, Very severe (Status asthmaticus or Respiratory failure)
d) Prediction of Postoperative Elective MV:
-PaCO₂ > 50 mmHg, PaO₂ < 50 mmHg, PFT < 50% of predicted
6-Risk assessment:
-Respiratory risk index score: predict postoperative respiratory complications or failure
b) Preparation:
1-Elective surgery should not take place in the presence of infection or untreated bronchospasm
2-Continue treatment: (Bronchodilators, Corticosteroids, …) up to the time of surgery
3-Pulmonary function tests and blood gases can be improved after the administration of bronchodilators
4-Stop smoking
5-Treatment of respiratory tract infection: Antibiotics after culture and sensitivity tests
6-Wait 2-3 weeks after clinical recovery of URTI (due to increased airway reactivity)
7-Treatment of complications if present: Dysrhythmia, Pulmonary HTN, Cor-pulmonale, Congestive heart failure
8-Chest physiotherapy: to remove secretions
9-Incentive spirometry: to improve lung expansion
10-Weight reduction of obese patients prepared for elective surgery
11-In severe cases it is important to assess the need for postoperative mechanical ventilation and reserve an ICU bed
c) Premedication:
1-Sedation:
-Anxiety may be a significant feature in the asthmatic patient, but a sedative premedication, such as an antihistaminics or benzodiazepine, is advantageous.
2-Anticholinergic:
-Atropine (to decrease bronchial secretions and vagal tone) 1-Atropine may be desirable for a smooth induction. It has a drying effect on secretions and can also improve dilatation in the larger bronchi by blocking vagal constrictor effects.
3-Corticosteroid Cover: if steroids have been used within the previous 3 months.
4-Avoid H2 blockers: lead to unopposed H1 bronchoconstriction.
Intraoperative:
a) Monitoring:
-ECG, NIBP, Pulse Oximeter, Capnography
-Pulsus paradoxus is present with Severe asthma & Tension pneumothorax (drop-in SBP ≥ 10 mmHg during inspiration) (Figure 2)
-Ventilator waveforms: Flow-Time scalar
-Spirometry: Volume-Pressure & Flow-Volume loops
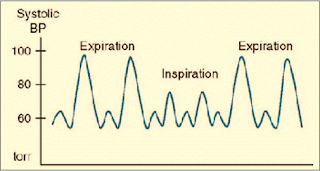 |
| Figure 2: Pulsus Paradoxus |
b) Precautions:
1-Avoid:
-β-blockers
-Histamine releasing drugs (Thiopental, Succinylcholine, Atracurium, Morphine, Meperidine)
2-Drug interactions:
-Ketamine + Theophylline → Seizures
-Halothane + (Aminophylline, β2-agonists, Hypercapnia) → Dysrhythmia
c) Regional anesthesia:
1-Avoid block above T6 as it leads to decreased Expiratory Reserve volume and decreased use of accessory m. → Ineffective cough and Retention of secretions → Postoperative respiratory complications.
2-Regional a. may be used for surgery or during labor. Epidural anesthesia could be safely administered to asthmatic parturients, even during acute exacerbations of asthma.
d) General anesthesia:
Induction:
1-Preoxygenation
2-Smooth induction, Warm, Humidified gases.
3-Blunt reflex bronchospasm induced by intubation (increase depth of anesthesia, Lidocaine, β2-agonist inhaler).
4-The use of IV lidocaine (1-1.5 mg/kg) before induction has been recommended, as it can prevent reflex bronchospasm, but not that resulting from the release of allergic mediators. However, topical lidocaine spray is not effective and can induce bronchoconstriction in asthmatic patients.
5-Bronchoconstriction can arise from the drug itself, or directly from tracheal intubation. Even when attacks are infrequent, tracheal intubation is one of the commonest causes of intraoperative bronchospasm in patients who have any history of asthma. The carina is particularly sensitive to stimulation.
6-Use of a laryngeal mask airway (LMA) has been shown to avoid the reversible bronchoconstriction associated with tracheal intubation.
Induction agents:
-Avoid Thiopental as it causes histamine release.
-Propofol, Ketamine, Etomidate, Benzodiazepines: are suitable agents.
-Ketamine: is a suitable induction agent for emergency anesthesia in asthmatics when a rapid sequence induction is required, as it has a protective effect against bronchospasm through sympathomimetic action by inhibition of noradrenaline reuptake, which was abolished by beta-adrenoceptor blockers.
-Inhalational induction:
-With Halothane or Sevoflurane in pediatric patients.
-With Sevoflurane can be given for emergency cesarean section in a patient with status asthmaticus.
Neuromuscular blockers:
-Succinylcholine: causes histamine release.
-Atracurium: constricts peripheral airways in doses that produce significant cardiovascular effects, and this probably results from histamine release which acts on H2 receptors. The peak effects occur 3 min. after a dose of 0.5 mg/kg.
-Cis-atracurium, Rocuronium, Vecuronium, and Pancuronium: are suitable agents.
Inhalational agents:
1-Avoid N₂O if there is large emphysematous bullae (rupture, tension pneumothorax) or pulmonary HTN (increased PVR → pulmonary edema).
2-Inhalational agents are potent bronchodilators, however, in severe asthma, they can worsen ventilation-perfusion inequalities and increase hypoxia, by reducing hypoxic pulmonary vasoconstriction.
3-Halothane can interact with Aminophylline to produce serious arrhythmias (ventricular fibrillation and ventricular tachycardia), even when theophylline levels are within the therapeutic range. Halothane also sensitizes the heart to the effect of exogenous and endogenous catecholamines.
4-Halothane, Isoflurane, and Enflurane are all effective at reversing antigen-induced bronchospasm. The effect of halothane is secondary to the blocking of baseline vagal tone.
5-The choice of Isoflurane, Sevoflurane, and Enflurane may therefore be more appropriate.
Controlled MV:
1-Use warmed, humidified gases
2-Parameters: VT: 10-15 ml/kg, RR: 6-10 b/min., decrease TI, increase TE, decrease inflating pressure
3-Avoid PEEP
Fluid management:
-Adequate hydration: (less viscid secretions)
Intraoperative Complications:
1-Bronchospasm: There is an increased sensitivity to airway manipulations during light anesthesia. Tracheal tube insertion can induce reversible bronchoconstriction (acute bronchospasm), triggered by mechanical stimuli, but not with an LMA.
Treatment of Acute Bronchospasm:
-Identify the cause
-If this occurs following tracheal intubation, the easiest initial maneuver is to deepen the anesthetic using a volatile agent or β2 agonist inhalation through the inspiratory limb of the breathing circuit or ETT adaptor (Figure 3)
 |
| Figure 3: Application of β2-agonist inhaler through ETT adaptor |
-IV Salbutamol 5-15 µg/kg in 10 ml saline over 10 min., for continued problems, a salbutamol infusion, 1-5 µg/kg/min. (with ECG monitoring)
-IV Aminophylline 5-6 mg/kg over in saline over 20 min. then 0.5-1 mg/kg/h. (with ECG monitoring)
N.B.1: Reduce aminophylline infusion rate by 30% because GA decreases hepatic blood flow by 30% and consequently aminophylline metabolism
N.B.2: Aminophylline has a narrow therapeutic index so, plasma theophylline levels must be measured if treatment is prolonged for more than 24 h. The therapeutic range is 10-20 mg/l but toxic effects such as fits, arrhythmias, and cardiac arrest have been described with plasma levels as low as 25 mg/l. Extreme caution is necessary if the patient has already been taking sustained-release theophylline preparations
-IV Hydrocortisone 1-2 mg/kg
-IV Ketamine in subanesthetic dose (bolus dose 0.75 mg/kg, infusion of 0.15 mg/kg/h) has been used to treat intractable bronchospasm
-IV Epinephrine 1 in 10 000 should be given in divided doses, 1-10 ml if there is complete airway closure. This may need to be repeated
2-Air trapping (Auto-PEEP, Intrinsic PEEP): due to decreased expiratory time, increased airway pressure, congested neck veins, decreased pulse pressure, hypotension due to decreased venous return and consequently cardiac output
3-Cardiac Dysrhythmias: can occur more frequently in the presence of hypoxia, hypercarbia, and acidosis, or following the overuse of sympathomimetic agents
4-Complications due to Drug interactions: mentioned before
5-Sudden Death: has been attributed to the combination of nebulized high dose β2-sympathomimetics and long-acting theophylline derivatives
The reverse of Neuromuscular blockers:
-Anticholinergic (Atropine) first then Cholinesterase inhibitor (Neostigmine)
Extubation:
-Deep extubation
-Awake extubation: blunt reflexes with IV lidocaine (1-1.5 mg/kg) first
Postoperative:
1-Monitoring, Semi sitting, Humidified O₂
2-Analgesia: NSAIDs (with caution), Local or regional a., Avoid Opioids can cause respiratory depression
3-Continue preoperative treatment
4-Avoidance of MV in a severe asthmatic was achieved by administration of a subanesthetic dose of halothane in 100% oxygen using a close-fitting mask
5-Elective MV if predicted preoperative or if indicated postoperative
Indications for Postoperative MV in Asthmatic Patients:
1-Distress and exhaustion
2-Deterioration in ABG. PaO₂ < 6.7 kPa (50 mmHg) or PaCO₂ > 6.7 kPa (50 mmHg), and increasing metabolic acidosis
3-Cardiac dysrhythmias or hypotension
4-Acute crises such as cardiorespiratory arrest, decreased conscious level due to sedatives, or a collapsed lung
5-Refractory asthma has been treated in the ICU with Magnesium sulphate, Halothane, and Propofol
Pierre Robin syndrome
By Author January 01, 2021
Pierre Robin syndrome:
➧ A rare syndrome in which there is a combination of:
-Severe micrognathia
-Posterior prolapse of the tongue
Other congenital abnormalities:
-Cleft palate
-Esophageal atresia
➧ The term syndrome is now reserved for those errors of morphogenesis with the simultaneous presence of multiple anomalies caused by a single etiology.
➧ The term sequence has been introduced to include any condition that includes a series of anomalies caused by a cascade of events initiated by a single malformation.
Pierre Robin sequence:
➧ The common features of which include:
1-Mandibular hypoplasia
2-Glossoptosis (Figure 1)
3-Incomplete cleft palate (Figure 2)
Although all three are not necessarily present.
This results in:
-Respiratory obstruction in infancy
-Failure to thrive
-Occasionally cor-pulmonale
 |
| Figure 1: Glossoptosis |
 |
| Figure 2: Incomplete cleft palate |
➧ The Robin sequence may be an isolated abnormality, or it may be part of a syndrome. There may be airway and intubation problems in any of these patients.
Associated syndromes:
➧ Pierre Robin sequence has been reported as occurring in association with:
-Stickler syndrome (20%-25% of these cases)
-Campomelic dysplasia
-Trisomy 11q syndrome
-Deletion 4q syndrome
-Velocardiofacial syndrome
-Treacher-Collins syndrome.
➧ These patients frequently require anesthesia at a young age.
➧ Management of long-term airway obstruction is a matter of debate, but at present tracheostomy seems to be back in favor. The mortality from pediatric tracheostomy has declined and it increases the safety of subsequent anesthetics.
Etiology:
➧ The exact causes of the Pierre Robin sequence are unknown. Possible mechanisms include:
-Genetic causes.
-Oligohydramnios, which may limit chin growth.
-Weakness of the facial muscles (myotonia).
-Connective tissue disease.
-Genetic causes.
-Oligohydramnios, which may limit chin growth.
-Weakness of the facial muscles (myotonia).
-Connective tissue disease.
➧ The genetic causes for some of the isolated cases (Pierre Robin sequence without any associated malformations) may include mutations or deletions of parts of the DNA neighboring the SOX9 gene (located in chromosome 17 (17q24)). This gene provides instructions for making protein SOX9 that regulates the activity of other genes, especially those involved in the development of the skeleton, including the jaw during embryonic development.
Preoperative abnormalities:
1. Many present as difficult or failed intubation during resuscitation at delivery. Hypoxic brain damage may be sustained at this stage.
2. The remainder usually presents within a few hours of birth when the micrognathia and glossoptosis cause breathing and feeding difficulties, with episodes of cyanosis when the child is in the supine position. Feeding difficulties correlate with the severity of airway obstruction. Subsequently, there is a failure to thrive.
2. The remainder usually presents within a few hours of birth when the micrognathia and glossoptosis cause breathing and feeding difficulties, with episodes of cyanosis when the child is in the supine position. Feeding difficulties correlate with the severity of airway obstruction. Subsequently, there is a failure to thrive.
3. There was a high incidence of concomitant problems, which included gastroesophageal reflux, congenital heart disease, and pulmonary disease.
4. Jaw index: A new index for defining micrognathia by measurement of three facial dimensions; children with Pierre Robin have an average index of more than 3.6 times the normal value.
5. Cleft palate occurs in 60%, and eye problems in 40% of Pierre Robin patients.
6. Chronic upper airway obstruction can result in cor-pulmonale. An increased pulmonary artery pressure may produce right-to-left shunting through a patent foramen ovale or a persistent ductus arteriosus.
7. Obstructive sleep apnea may occur and managed by the use of nasal CPAP.
Airway obstruction management:
➧ A sequence of strategies is recommended in an attempt to minimize airway obstruction and allow safe feeding. The treatment required depends upon severity:
-Initially, the neonate is nursed in the prone position. If this fails, prolonged nasopharyngeal intubation may help to protect the airway.
-If respiratory distress and failure to thrive persist, and a lateral X-ray of the neck in the supine position shows upper airway obstruction, suturing of the tongue to the lower gum or lip (tongue-to-lip adhesion) may be needed. Modified nasopharyngeal tubes or splints have been described. Feeding may be undertaken via a nasogastric or a gastrostomy tube.
-Respiration and oxygen saturation are monitored, and appropriate oxygen supplementation is given. Sometimes tracheostomy may be required, although previously there has been a reluctance to resort to this.
-Benjamin and Walker (1991), classified them into three groups according to the treatment required:
1-Mild group (needing posture alone)
2-Moderate group (needing nasopharyngeal tube)
3-Severe group (needing tracheal intubation or tracheostomy).
-All deaths can occur in the latter group, from hypoxic brain damage at birth.
-As the child grows, the obstruction tends to improve, partly from the growth of the mandible and the size of the airway, and partly as a result of better neurological control of the tongue muscles.
-Problems mainly seem to resolve by the time the child is 6 months old:
-Mild cases could be nursed supine from 3 to 6 months.
In the moderate group, nasopharyngeal intubation is required for between 14 days and 14 weeks, after which they were nursed prone.
-All could sleep supine by 6 months.
Anesthetic problems:
1. Even in the un-anesthetized infant, during the first few months of life, respiratory obstruction occurs in the supine position. The main mechanism for this is thought to be glossoptosis, prolapse of the tongue backward, but it is now realized that there are multiple factors.
➧ It is believed that obstruction is related to a combination of the anatomical abnormalities of the mandible with functional impairment of the genioglossus and other pharyngeal muscles, that are concerned with the maintenance of the airway.
➧ Varying degrees of obstruction exist, ranging from none at all to obstruction when the neonate is asleep and, in the worst cases, obstruction in the awake state. In any neonate, obstruction worsens during an upper respiratory tract infection, feeding, and crying.
➧ It is suggested that the site of obstruction varies from patient to patient. From endoscopic observations, these have been classified into:
-Type 1: A true glossoptosis in which the dorsum of the tongue is as opposed to the posterior pharyngeal wall.
-Type 2: The tongue compresses the soft palate against the posterior pharyngeal wall so that all three structures meet in the upper oropharynx.
-Type 3: Medial apposition of the lateral pharyngeal walls.
-Type 4: A sphincteric constriction of the pharynx.
2. Oxygen desaturation and obstructive sleep apnea, detected by pulse oximetry and polysomnography, occur in the majority of neonates and contribute to mortality from obstruction.
3. Gastro-esophageal reflux may be present.
4. The unusual facial configuration, in particular the receding lower jaw, makes it difficult to maintain an airtight fit with an anesthetic mask.
5. Difficult or failed intubation results from a combination of micrognathia, and prolapse or inward sucking of the posteriorly attached, and often enlarged, tongue. This may be compounded by the presence of a tongue tie which, paradoxically, may prevent airway obstruction.
So, intubation problems can be underestimated because of the lack of preoperative airway obstruction. However, once the tongue tie had been corrected, subsequent intubation became easy.
So, intubation problems can be underestimated because of the lack of preoperative airway obstruction. However, once the tongue tie had been corrected, subsequent intubation became easy.
6. Pulmonary edema can occur after relief of airway obstruction following palatal repair of cleft palate.
Airway management:
➧ Monitoring by pulse oximetry, to detect airway obstruction, is crucial.
➧ Several methods have been proposed to overcome the problem of difficult intubation, some under general anesthesia, and some in awake patients. The consensus of opinion now seems to favor awake techniques.
1-Asleep technique with 'Jackson anterior commissure laryngoscope': (Figure 3)
➧ Handler and Keon (1983) described a technique for intubation for the anesthetized spontaneously breathing patient, using 'Jackson anterior commissure laryngoscope':
 |
| Figure 3: Jackson anterior commissure laryngoscope |
-The head is elevated above the shoulders, with flexion of the lower cervical vertebrae and extension at the atlanto-occipital joint.
-The laryngoscope is introduced into the right side of the mouth. Only the tip is directed towards the midline, the proximal end remaining laterally so that a further 30 degrees of anterior angulation can be obtained.
-The laryngoscope is introduced into the right side of the mouth. Only the tip is directed towards the midline, the proximal end remaining laterally so that a further 30 degrees of anterior angulation can be obtained.
-The narrow, closed blade prevents the tongue from falling in and obscuring the view of the larynx. When visualized, the epiglottis is elevated, and the larynx is entered.
-Intubation is then achieved by passing a lubricated tube, without its adaptor, down the laryngoscope. It is held in place with 'alligator forceps' whilst the laryngoscope is withdrawn.
-Intubation is then achieved by passing a lubricated tube, without its adaptor, down the laryngoscope. It is held in place with 'alligator forceps' whilst the laryngoscope is withdrawn.
2-Asleep technique with blind nasal intubation in the prone position:
➧ The prone position avoids the problems in the supine position, this position allows the tongue and mandible to fall forward under the effect of gravity and leave the larynx exposed.
3-Fibreoptic bronchoscopic techniques:
➧ In small infants, the 'tube over bronchoscope' technique is not always possible because of the small size of the tube, therefore a 'Seldinger technique' may be necessary:
-After the administration of atropine, ketamine IM, and topical lidocaine, a fibreoptic bronchoscope (OD 3.6 mm, L 60 cm, and suction channel 1.2 mm) is passed through one nostril.
-The tongue is held forward with 'Magill forceps', until the vocal cords are seen, but not entered, because of the risk of total obstruction.
-Under direct vision, a 'Teflon-coated guidewire' with a flexible tip is passed via the suction channel into the trachea.
-The bronchoscope is carefully removed leaving the wire in place, and an ID 3 mm nasotracheal tube is then passed over it into the trachea.
➧ Pediatric bronchoscopes of 2.5 mm diameter are now available, but their very fineness makes them less easy to handle than the 4 mm bronchoscopes).
4-Awake techniques using 'Holiger pediatric anterior commissure laryngoscope': (Figure 4)
 |
| Figure 4: Holiger anterior commissure laryngoscope |
5-Laryngeal mask airway (LMA) techniques:
➧ Placement of LMA following topical anesthesia in awake infants and the use of LMA to guide an introducer for subsequent intubation can be used in an emergency, and electively.
6-The use of a lighted stylet:
Read more ☛ Treacher Collins Syndrome
Followers
Featured Post
Popular Posts
-
Airway Blocks 1-Superior laryngeal n. block: Block of the superior laryngeal nerve can provide anesthesia of the larynx from the epiglottis ...
-
Awareness during Anesthesia Groups at risk for awareness: Awareness is due to too light plane of anesthesia. It is more likely where mus...
-
Accidental Total Spinal Anesthesia Definition: ➧ A syndrome of the central neurological blockade. ➧ It occurs when a vol...
-
Anesthetic Considerations for Patients with Liver Disease Preoperative: 1. Assess the Degree of hepatic impairment, Severity, and Hepati...
-
Failed Spinal Anesthesia Introduction: ➧ Spinal (intrathecal) anesthesia is one of the most reliable regional block methods: the needle...
-
Laryngeal Mask Airway (LMA) 1-The LMA-Classic™: -It is a reusable LMA™ airway for general anesthesia. -The LMA-Classic™ is available in...
-
IV Fluids A) Crystalloid solutions (< 30 000 Dalton): 1-Hypotonic solutions: - D 5 W, ½ NS, D5 ¼ NS, D5 ½ NS. 2-Isotonic solutio...
-
Dexmedetomidine Mechanism of Action: -It is an imidazole derivative and is a specific alpha-2 adrenoceptor agonist that acts via post-sy...
-
Methods to stabilize ETT intracuff pressure -The use of N₂O, which is well-known to diffuse into ETT cuffs, and the lack of frequent cont...
-
Fat Embolism Syndrome (FES) ➧ Fat embolism can be difficult to diagnose. It most often follows a closed fracture of a long bone but there...