Categories
- Airway
- Anesthesia machine
- Anesthetic drugs
- CNS diseases
- Complications in anesthesia
- Congenital cardiovascular diseases
- Endocine Emergencies
- Fluid & Electrolyte disturbances
- GIT diseases
- ICU
- Inborn errors of metabolism
- Infections
- Monitoring
- Neuropsychiatric diseases
- Nutritional
- Obstetric anesthesia
- Ophthalmic anesthesia
- Otorhinolaryngology
- Pediatric anesthesia
- Plastic
- Regional anesthesia
- Renal diseases
- Research
- Respiratory diseases
- Skin & Musculoskeletal diseases
- Thoracic surgery
Bronchial Asthma
By Author January 01, 2021
Anesthetic Management of Pt. with Bronchial Asthma
Definition:
➧ It is an acute obstructive pulmonary disease. It is due to the hyperreactivity of the tracheobronchial tree, in which some exogenous or endogenous stimuli can produce reversible airway obstruction.
➧ Histamine and the leukotrienes are the most active chemical mediators, while acetylcholine contributes via a disturbance in autonomic balance by stimulation of M3 cholinergic receptors on bronchial smooth muscles causing bronchospasm.
Pathological changes: (Figure 1)
-Prolonged expiration
-Increased residual volume and functional residual capacity
-Increased work on breathing
-Reduced vital capacity, inspiratory capacity, and expiratory reserve volume
-Ventilation-perfusion inequalities
-Hypoxia
-Bronchospasm
-Mucus secretion
-Edema of the bronchial mucosa
-Epithelial desquamation
 |
| Figure 1: Pathological changes in Br. Asthma |
Anesthetic Management:
Preoperative:
a) Assessment:
1-History:
-Wheezing, dyspnea, cough, and sputum production.
2-Classification of Asthma:
-Mild Intermittent: Mild symptoms up to two days a week and up to two nights a month
-Mild Persistent: Symptoms more than twice a week, but no more than once in a single day
-Moderate Persistent: Symptoms once a day and more than one night a week
-Severe Persistent: Symptoms throughout the day on most days and frequently at night
3-Drug Therapy:
-β2-adrenoceptor agonists (albuterol, levalbuterol, salbutamol, terbutaline, salmeterol, formoterol)
-Xanthine derivatives (theophylline, aminophylline)
-Anticholinergics (Ipratropium bromide)
-Corticosteroids (beclometasone, budesonide, fluticasone, flunisolide, ciclesonide, mometasone).
-Mast cell stabilizers (sodium cromoglycate, nedocromil sodium)
-Leukotriene modifiers (montelukast, pranlukast, zafirlukast, zileuton)
-Allergy medications (allergy shots (immunotherapy), omalizumab)
4-Examination:
-Pulsus paradoxus
-Chest auscultation: The chest may be hyper resonant, with diminished breath sounds, prolonged expiration, and audible wheezes. In very severe cases the wheezes disappear
5-Investigations:
1. CBC:
-Polycythemia (if there is chronic hypoxemia)
-Eosinophilia: indicates the degree of airway inflammation
-Neutrophilia: bacterial infection
-Lymphocytosis: viral infection
2. Chest X-ray (CXR):
-Baseline for postoperative follow up
-May show an increase in bronchovascular markings and hyperinflation
-In the later stages, may show some degree of emphysema
-In an acute asthma attack, a CXR is essential to exclude pneumothorax
3. Arterial Blood Gases (ABG):
-Hypoxemia, Hypercarbia
4. Pulmonary Function Tests (PFT):
a) Tests indicate Severe Obstruction:
-Peak Expiratory Flow Rate (PEFR) of less than 120 l/min.
-Maximum Voluntary Ventilation (MVV) of less than 50% of predicted
b) Tests indicate the need for Postoperative Mechanical Ventilation (MV):
-FEV1 of less than 1 liter
-FEV1/VC ratio of less than 40% of predicted
-Increased PaCO₂ > 50 mmHg
c) Assessment of Severity of Asthma by PFT:
-Mild, Moderate, Severe, Very severe (Status asthmaticus or Respiratory failure)
d) Prediction of Postoperative Elective MV:
-PaCO₂ > 50 mmHg, PaO₂ < 50 mmHg, PFT < 50% of predicted
6-Risk assessment:
-Respiratory risk index score: predict postoperative respiratory complications or failure
b) Preparation:
1-Elective surgery should not take place in the presence of infection or untreated bronchospasm
2-Continue treatment: (Bronchodilators, Corticosteroids, …) up to the time of surgery
3-Pulmonary function tests and blood gases can be improved after the administration of bronchodilators
4-Stop smoking
5-Treatment of respiratory tract infection: Antibiotics after culture and sensitivity tests
6-Wait 2-3 weeks after clinical recovery of URTI (due to increased airway reactivity)
7-Treatment of complications if present: Dysrhythmia, Pulmonary HTN, Cor-pulmonale, Congestive heart failure
8-Chest physiotherapy: to remove secretions
9-Incentive spirometry: to improve lung expansion
10-Weight reduction of obese patients prepared for elective surgery
11-In severe cases it is important to assess the need for postoperative mechanical ventilation and reserve an ICU bed
c) Premedication:
1-Sedation:
-Anxiety may be a significant feature in the asthmatic patient, but a sedative premedication, such as an antihistaminics or benzodiazepine, is advantageous.
2-Anticholinergic:
-Atropine (to decrease bronchial secretions and vagal tone) 1-Atropine may be desirable for a smooth induction. It has a drying effect on secretions and can also improve dilatation in the larger bronchi by blocking vagal constrictor effects.
3-Corticosteroid Cover: if steroids have been used within the previous 3 months.
4-Avoid H2 blockers: lead to unopposed H1 bronchoconstriction.
Intraoperative:
a) Monitoring:
-ECG, NIBP, Pulse Oximeter, Capnography
-Pulsus paradoxus is present with Severe asthma & Tension pneumothorax (drop-in SBP ≥ 10 mmHg during inspiration) (Figure 2)
-Ventilator waveforms: Flow-Time scalar
-Spirometry: Volume-Pressure & Flow-Volume loops
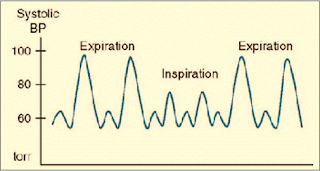 |
| Figure 2: Pulsus Paradoxus |
b) Precautions:
1-Avoid:
-β-blockers
-Histamine releasing drugs (Thiopental, Succinylcholine, Atracurium, Morphine, Meperidine)
2-Drug interactions:
-Ketamine + Theophylline → Seizures
-Halothane + (Aminophylline, β2-agonists, Hypercapnia) → Dysrhythmia
c) Regional anesthesia:
1-Avoid block above T6 as it leads to decreased Expiratory Reserve volume and decreased use of accessory m. → Ineffective cough and Retention of secretions → Postoperative respiratory complications.
2-Regional a. may be used for surgery or during labor. Epidural anesthesia could be safely administered to asthmatic parturients, even during acute exacerbations of asthma.
d) General anesthesia:
Induction:
1-Preoxygenation
2-Smooth induction, Warm, Humidified gases.
3-Blunt reflex bronchospasm induced by intubation (increase depth of anesthesia, Lidocaine, β2-agonist inhaler).
4-The use of IV lidocaine (1-1.5 mg/kg) before induction has been recommended, as it can prevent reflex bronchospasm, but not that resulting from the release of allergic mediators. However, topical lidocaine spray is not effective and can induce bronchoconstriction in asthmatic patients.
5-Bronchoconstriction can arise from the drug itself, or directly from tracheal intubation. Even when attacks are infrequent, tracheal intubation is one of the commonest causes of intraoperative bronchospasm in patients who have any history of asthma. The carina is particularly sensitive to stimulation.
6-Use of a laryngeal mask airway (LMA) has been shown to avoid the reversible bronchoconstriction associated with tracheal intubation.
Induction agents:
-Avoid Thiopental as it causes histamine release.
-Propofol, Ketamine, Etomidate, Benzodiazepines: are suitable agents.
-Ketamine: is a suitable induction agent for emergency anesthesia in asthmatics when a rapid sequence induction is required, as it has a protective effect against bronchospasm through sympathomimetic action by inhibition of noradrenaline reuptake, which was abolished by beta-adrenoceptor blockers.
-Inhalational induction:
-With Halothane or Sevoflurane in pediatric patients.
-With Sevoflurane can be given for emergency cesarean section in a patient with status asthmaticus.
Neuromuscular blockers:
-Succinylcholine: causes histamine release.
-Atracurium: constricts peripheral airways in doses that produce significant cardiovascular effects, and this probably results from histamine release which acts on H2 receptors. The peak effects occur 3 min. after a dose of 0.5 mg/kg.
-Cis-atracurium, Rocuronium, Vecuronium, and Pancuronium: are suitable agents.
Inhalational agents:
1-Avoid N₂O if there is large emphysematous bullae (rupture, tension pneumothorax) or pulmonary HTN (increased PVR → pulmonary edema).
2-Inhalational agents are potent bronchodilators, however, in severe asthma, they can worsen ventilation-perfusion inequalities and increase hypoxia, by reducing hypoxic pulmonary vasoconstriction.
3-Halothane can interact with Aminophylline to produce serious arrhythmias (ventricular fibrillation and ventricular tachycardia), even when theophylline levels are within the therapeutic range. Halothane also sensitizes the heart to the effect of exogenous and endogenous catecholamines.
4-Halothane, Isoflurane, and Enflurane are all effective at reversing antigen-induced bronchospasm. The effect of halothane is secondary to the blocking of baseline vagal tone.
5-The choice of Isoflurane, Sevoflurane, and Enflurane may therefore be more appropriate.
Controlled MV:
1-Use warmed, humidified gases
2-Parameters: VT: 10-15 ml/kg, RR: 6-10 b/min., decrease TI, increase TE, decrease inflating pressure
3-Avoid PEEP
Fluid management:
-Adequate hydration: (less viscid secretions)
Intraoperative Complications:
1-Bronchospasm: There is an increased sensitivity to airway manipulations during light anesthesia. Tracheal tube insertion can induce reversible bronchoconstriction (acute bronchospasm), triggered by mechanical stimuli, but not with an LMA.
Treatment of Acute Bronchospasm:
-Identify the cause
-If this occurs following tracheal intubation, the easiest initial maneuver is to deepen the anesthetic using a volatile agent or β2 agonist inhalation through the inspiratory limb of the breathing circuit or ETT adaptor (Figure 3)
 |
| Figure 3: Application of β2-agonist inhaler through ETT adaptor |
-IV Salbutamol 5-15 µg/kg in 10 ml saline over 10 min., for continued problems, a salbutamol infusion, 1-5 µg/kg/min. (with ECG monitoring)
-IV Aminophylline 5-6 mg/kg over in saline over 20 min. then 0.5-1 mg/kg/h. (with ECG monitoring)
N.B.1: Reduce aminophylline infusion rate by 30% because GA decreases hepatic blood flow by 30% and consequently aminophylline metabolism
N.B.2: Aminophylline has a narrow therapeutic index so, plasma theophylline levels must be measured if treatment is prolonged for more than 24 h. The therapeutic range is 10-20 mg/l but toxic effects such as fits, arrhythmias, and cardiac arrest have been described with plasma levels as low as 25 mg/l. Extreme caution is necessary if the patient has already been taking sustained-release theophylline preparations
-IV Hydrocortisone 1-2 mg/kg
-IV Ketamine in subanesthetic dose (bolus dose 0.75 mg/kg, infusion of 0.15 mg/kg/h) has been used to treat intractable bronchospasm
-IV Epinephrine 1 in 10 000 should be given in divided doses, 1-10 ml if there is complete airway closure. This may need to be repeated
2-Air trapping (Auto-PEEP, Intrinsic PEEP): due to decreased expiratory time, increased airway pressure, congested neck veins, decreased pulse pressure, hypotension due to decreased venous return and consequently cardiac output
3-Cardiac Dysrhythmias: can occur more frequently in the presence of hypoxia, hypercarbia, and acidosis, or following the overuse of sympathomimetic agents
4-Complications due to Drug interactions: mentioned before
5-Sudden Death: has been attributed to the combination of nebulized high dose β2-sympathomimetics and long-acting theophylline derivatives
The reverse of Neuromuscular blockers:
-Anticholinergic (Atropine) first then Cholinesterase inhibitor (Neostigmine)
Extubation:
-Deep extubation
-Awake extubation: blunt reflexes with IV lidocaine (1-1.5 mg/kg) first
Postoperative:
1-Monitoring, Semi sitting, Humidified O₂
2-Analgesia: NSAIDs (with caution), Local or regional a., Avoid Opioids can cause respiratory depression
3-Continue preoperative treatment
4-Avoidance of MV in a severe asthmatic was achieved by administration of a subanesthetic dose of halothane in 100% oxygen using a close-fitting mask
5-Elective MV if predicted preoperative or if indicated postoperative
Indications for Postoperative MV in Asthmatic Patients:
1-Distress and exhaustion
2-Deterioration in ABG. PaO₂ < 6.7 kPa (50 mmHg) or PaCO₂ > 6.7 kPa (50 mmHg), and increasing metabolic acidosis
3-Cardiac dysrhythmias or hypotension
4-Acute crises such as cardiorespiratory arrest, decreased conscious level due to sedatives, or a collapsed lung
5-Refractory asthma has been treated in the ICU with Magnesium sulphate, Halothane, and Propofol
Pierre Robin syndrome
By Author January 01, 2021
Pierre Robin syndrome:
➧ A rare syndrome in which there is a combination of:
-Severe micrognathia
-Posterior prolapse of the tongue
Other congenital abnormalities:
-Cleft palate
-Esophageal atresia
➧ The term syndrome is now reserved for those errors of morphogenesis with the simultaneous presence of multiple anomalies caused by a single etiology.
➧ The term sequence has been introduced to include any condition that includes a series of anomalies caused by a cascade of events initiated by a single malformation.
Pierre Robin sequence:
➧ The common features of which include:
1-Mandibular hypoplasia
2-Glossoptosis (Figure 1)
3-Incomplete cleft palate (Figure 2)
Although all three are not necessarily present.
This results in:
-Respiratory obstruction in infancy
-Failure to thrive
-Occasionally cor-pulmonale
 |
| Figure 1: Glossoptosis |
 |
| Figure 2: Incomplete cleft palate |
➧ The Robin sequence may be an isolated abnormality, or it may be part of a syndrome. There may be airway and intubation problems in any of these patients.
Associated syndromes:
➧ Pierre Robin sequence has been reported as occurring in association with:
-Stickler syndrome (20%-25% of these cases)
-Campomelic dysplasia
-Trisomy 11q syndrome
-Deletion 4q syndrome
-Velocardiofacial syndrome
-Treacher-Collins syndrome.
➧ These patients frequently require anesthesia at a young age.
➧ Management of long-term airway obstruction is a matter of debate, but at present tracheostomy seems to be back in favor. The mortality from pediatric tracheostomy has declined and it increases the safety of subsequent anesthetics.
Etiology:
➧ The exact causes of the Pierre Robin sequence are unknown. Possible mechanisms include:
-Genetic causes.
-Oligohydramnios, which may limit chin growth.
-Weakness of the facial muscles (myotonia).
-Connective tissue disease.
-Genetic causes.
-Oligohydramnios, which may limit chin growth.
-Weakness of the facial muscles (myotonia).
-Connective tissue disease.
➧ The genetic causes for some of the isolated cases (Pierre Robin sequence without any associated malformations) may include mutations or deletions of parts of the DNA neighboring the SOX9 gene (located in chromosome 17 (17q24)). This gene provides instructions for making protein SOX9 that regulates the activity of other genes, especially those involved in the development of the skeleton, including the jaw during embryonic development.
Preoperative abnormalities:
1. Many present as difficult or failed intubation during resuscitation at delivery. Hypoxic brain damage may be sustained at this stage.
2. The remainder usually presents within a few hours of birth when the micrognathia and glossoptosis cause breathing and feeding difficulties, with episodes of cyanosis when the child is in the supine position. Feeding difficulties correlate with the severity of airway obstruction. Subsequently, there is a failure to thrive.
2. The remainder usually presents within a few hours of birth when the micrognathia and glossoptosis cause breathing and feeding difficulties, with episodes of cyanosis when the child is in the supine position. Feeding difficulties correlate with the severity of airway obstruction. Subsequently, there is a failure to thrive.
3. There was a high incidence of concomitant problems, which included gastroesophageal reflux, congenital heart disease, and pulmonary disease.
4. Jaw index: A new index for defining micrognathia by measurement of three facial dimensions; children with Pierre Robin have an average index of more than 3.6 times the normal value.
5. Cleft palate occurs in 60%, and eye problems in 40% of Pierre Robin patients.
6. Chronic upper airway obstruction can result in cor-pulmonale. An increased pulmonary artery pressure may produce right-to-left shunting through a patent foramen ovale or a persistent ductus arteriosus.
7. Obstructive sleep apnea may occur and managed by the use of nasal CPAP.
Airway obstruction management:
➧ A sequence of strategies is recommended in an attempt to minimize airway obstruction and allow safe feeding. The treatment required depends upon severity:
-Initially, the neonate is nursed in the prone position. If this fails, prolonged nasopharyngeal intubation may help to protect the airway.
-If respiratory distress and failure to thrive persist, and a lateral X-ray of the neck in the supine position shows upper airway obstruction, suturing of the tongue to the lower gum or lip (tongue-to-lip adhesion) may be needed. Modified nasopharyngeal tubes or splints have been described. Feeding may be undertaken via a nasogastric or a gastrostomy tube.
-Respiration and oxygen saturation are monitored, and appropriate oxygen supplementation is given. Sometimes tracheostomy may be required, although previously there has been a reluctance to resort to this.
-Benjamin and Walker (1991), classified them into three groups according to the treatment required:
1-Mild group (needing posture alone)
2-Moderate group (needing nasopharyngeal tube)
3-Severe group (needing tracheal intubation or tracheostomy).
-All deaths can occur in the latter group, from hypoxic brain damage at birth.
-As the child grows, the obstruction tends to improve, partly from the growth of the mandible and the size of the airway, and partly as a result of better neurological control of the tongue muscles.
-Problems mainly seem to resolve by the time the child is 6 months old:
-Mild cases could be nursed supine from 3 to 6 months.
In the moderate group, nasopharyngeal intubation is required for between 14 days and 14 weeks, after which they were nursed prone.
-All could sleep supine by 6 months.
Anesthetic problems:
1. Even in the un-anesthetized infant, during the first few months of life, respiratory obstruction occurs in the supine position. The main mechanism for this is thought to be glossoptosis, prolapse of the tongue backward, but it is now realized that there are multiple factors.
➧ It is believed that obstruction is related to a combination of the anatomical abnormalities of the mandible with functional impairment of the genioglossus and other pharyngeal muscles, that are concerned with the maintenance of the airway.
➧ Varying degrees of obstruction exist, ranging from none at all to obstruction when the neonate is asleep and, in the worst cases, obstruction in the awake state. In any neonate, obstruction worsens during an upper respiratory tract infection, feeding, and crying.
➧ It is suggested that the site of obstruction varies from patient to patient. From endoscopic observations, these have been classified into:
-Type 1: A true glossoptosis in which the dorsum of the tongue is as opposed to the posterior pharyngeal wall.
-Type 2: The tongue compresses the soft palate against the posterior pharyngeal wall so that all three structures meet in the upper oropharynx.
-Type 3: Medial apposition of the lateral pharyngeal walls.
-Type 4: A sphincteric constriction of the pharynx.
2. Oxygen desaturation and obstructive sleep apnea, detected by pulse oximetry and polysomnography, occur in the majority of neonates and contribute to mortality from obstruction.
3. Gastro-esophageal reflux may be present.
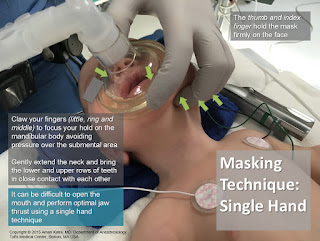
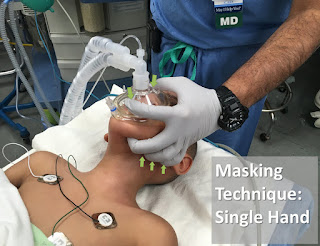
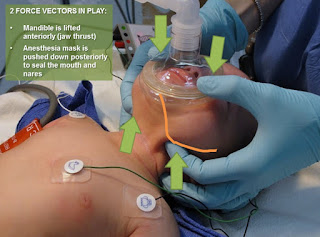
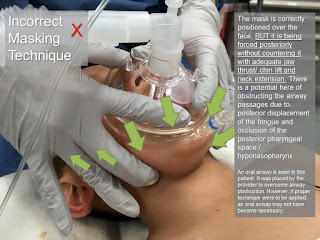
4. The unusual facial configuration, in particular the receding lower jaw, makes it difficult to maintain an airtight fit with an anesthetic mask.
5. Difficult or failed intubation results from a combination of micrognathia, and prolapse or inward sucking of the posteriorly attached, and often enlarged, tongue. This may be compounded by the presence of a tongue tie which, paradoxically, may prevent airway obstruction.
So, intubation problems can be underestimated because of the lack of preoperative airway obstruction. However, once the tongue tie had been corrected, subsequent intubation became easy.
So, intubation problems can be underestimated because of the lack of preoperative airway obstruction. However, once the tongue tie had been corrected, subsequent intubation became easy.
6. Pulmonary edema can occur after relief of airway obstruction following palatal repair of cleft palate.
Airway management:
➧ Monitoring by pulse oximetry, to detect airway obstruction, is crucial.
➧ Several methods have been proposed to overcome the problem of difficult intubation, some under general anesthesia, and some in awake patients. The consensus of opinion now seems to favor awake techniques.
1-Asleep technique with 'Jackson anterior commissure laryngoscope': (Figure 3)
➧ Handler and Keon (1983) described a technique for intubation for the anesthetized spontaneously breathing patient, using 'Jackson anterior commissure laryngoscope':
 |
| Figure 3: Jackson anterior commissure laryngoscope |
-The head is elevated above the shoulders, with flexion of the lower cervical vertebrae and extension at the atlanto-occipital joint.
-The laryngoscope is introduced into the right side of the mouth. Only the tip is directed towards the midline, the proximal end remaining laterally so that a further 30 degrees of anterior angulation can be obtained.
-The laryngoscope is introduced into the right side of the mouth. Only the tip is directed towards the midline, the proximal end remaining laterally so that a further 30 degrees of anterior angulation can be obtained.
-The narrow, closed blade prevents the tongue from falling in and obscuring the view of the larynx. When visualized, the epiglottis is elevated, and the larynx is entered.
-Intubation is then achieved by passing a lubricated tube, without its adaptor, down the laryngoscope. It is held in place with 'alligator forceps' whilst the laryngoscope is withdrawn.
-Intubation is then achieved by passing a lubricated tube, without its adaptor, down the laryngoscope. It is held in place with 'alligator forceps' whilst the laryngoscope is withdrawn.
2-Asleep technique with blind nasal intubation in the prone position:
➧ The prone position avoids the problems in the supine position, this position allows the tongue and mandible to fall forward under the effect of gravity and leave the larynx exposed.
3-Fibreoptic bronchoscopic techniques:
➧ In small infants, the 'tube over bronchoscope' technique is not always possible because of the small size of the tube, therefore a 'Seldinger technique' may be necessary:
-After the administration of atropine, ketamine IM, and topical lidocaine, a fibreoptic bronchoscope (OD 3.6 mm, L 60 cm, and suction channel 1.2 mm) is passed through one nostril.
-The tongue is held forward with 'Magill forceps', until the vocal cords are seen, but not entered, because of the risk of total obstruction.
-Under direct vision, a 'Teflon-coated guidewire' with a flexible tip is passed via the suction channel into the trachea.
-The bronchoscope is carefully removed leaving the wire in place, and an ID 3 mm nasotracheal tube is then passed over it into the trachea.
➧ Pediatric bronchoscopes of 2.5 mm diameter are now available, but their very fineness makes them less easy to handle than the 4 mm bronchoscopes).
4-Awake techniques using 'Holiger pediatric anterior commissure laryngoscope': (Figure 4)
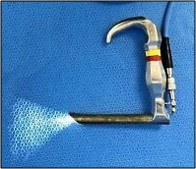 |
| Figure 4: Holiger anterior commissure laryngoscope |
5-Laryngeal mask airway (LMA) techniques:
➧ Placement of LMA following topical anesthesia in awake infants and the use of LMA to guide an introducer for subsequent intubation can be used in an emergency, and electively.
6-The use of a lighted stylet:
Read more ☛ Treacher Collins Syndrome
Anesthetic Precautions for Bloody and Lengthy Surgery
By Author October 01, 2020
Anesthetic Precautions for Bloody and Lengthy Surgery
A) Precautions for Bloody Surgery
1-Decrease blood loss by:
➧ The surgical site elevation is 10-15°
➧ Use of tourniquet
➧ Local infiltration of epinephrine
➧ Use of topical hemostats
➧ Application of hypotensive anesthesia
➧ Controlled mechanical ventilation (decrease venous return → decrease Cardiac output and PaCO₂)
➧ Use antifibrinolytic agents: (Aprotinin, Epsilon Amino Caproic Acid, Tranexamic acid)
➧ Use desmopressin (DDAVP)
➧ Keep patient normothermic
➧ Give IV Calcium (to prevent citrate toxicity and help coagulation)
➧ Restrict diagnostic phlebotomies
➧ Avoid: (Atropine, Ketamine, Pancuronium) (increase: Heart rate, Blood pressure, Endogenous catecholamines)
2-Restore Blood loss rapidly by:
➧ Prepare type-specific, cross-matched blood
➧ Preoperative autologous blood donation
➧ Apply normovolemic hemodilution
➧ Use cell saver devices (Blood Salvage)
➧ Insert wide bore IV cannulae
➧ Use blood substitutes
➧ Use rapid infusion devices
➧ Use blood warmers
B) Precautions for Lengthy Surgery
1-Decrease Hypothermia by:
➧ Monitoring by a temperature probe
➧ Increase ambient room temp. ≥ 24° (in Adults), ≥ 26° (in Pediatrics)
➧ Cotton wrapping of the limbs and head
➧ Use a warming blanket/mattress
➧ Warm IV fluids
➧ Warm irrigating fluids
➧ Warm humidified inspired gases
➧ Use low-flow anesthesia
➧ Use blood warmers
2-DVT prophylaxis.
3-Pressure sore prophylaxis (Padding of pressure points).
4-Eye protection (Tap and Pad).
5-Invasive monitoring (CVP, IBP).
6-Avoid N₂O (causes: Bone marrow depression, Megaloblastic anemia, Agranulocytosis, Peripheral neuritis, Immune response depression).
7-Use Isoflurane (More rapid recovery).
8-Insert nasogastric tube (to avoid gastric distension).
9-Use high volume, low-pressure ETT cuff (with frequent monitoring of intracuff pressure or use intracuff saline).
10-Avoid hypovolemia (by: Monitoring, Fluid chart, IV fluids).
Minimally Invasive and Non-invasive Cardiac Output Monitoring
By Author October 01, 2020
Minimally Invasive and Non-invasive Cardiac Output Monitoring
➧ The concept of determining blood flow/time Cardiac Output (CO) by measuring the dilution of a ‘known substance’ in the blood (Fick’s principle) has been applied by pulmonary artery (PA) catheter using the thermodilution technique remains the ‘Gold standard’ approach of CO monitoring. However, it is not without risk.
I. Esophageal Doppler U/S:
Principle:
➧ It measures blood flow velocity in the descending thoracic aorta by using the change in frequency of the U/S beam as it reflects off a moving object (Doppler shift).
➧ If this measurement is combined with an estimate of the cross-sectional area of the aorta (derived value from pt. age, height & weight using nomograms), it allows hemodynamic variables to be calculated [Stroke Volume (SV), CO, Cardiac Index (CI)].
Advantage:
➧ Provides continuous measurements
Limitations:
➧ The following three conditions must be met to guarantee accuracy:
1-The cross-sectional area must be accurate.
2-The US beam must be directed parallel to the flow of blood
3-The beam direction cannot move to any great degree between measurements.
➧ Variations in the above conditions lead to inaccuracies.
Disadvantages:
➧ The main problem with its use as a continuous CO monitor relates to its precision which indicates the reproducibility of a measurement.
➧ It is operator-dependent and it is very easy for the position of the probe to change between measurements which will reduce the precision.
➧ The need for frequent repositioning is not well tolerated by an awake pt. and is therefore need sedation.
II. Echocardiography: [Trans-Thoracic & Trans-Esophageal (TEE)]
Principle:
➧ This technique can be used to calculate SV which can then be multiplied by HR to give the CO.
➧ For the assessment of SV; 2 steps are necessary:
1- Calculation of flow velocity from the area under the Doppler velocity wave. This represents the distance RBCs are projected forward in one cardiac cycle.
2- Determination of area through which the flow is pushed forward (calculated from the diameter assuming a circular shape or determined by direct planimetry). Measurements can be performed at the level of [PA, Mitral Valve (MV), or Aortic Valve (AV)].
Advantage:
➧ Good correlation with thermodilution CO measurements providing that the MV is competent.
Limitations:
➧ It is very difficult to measure the diameter of PA.
➧ Measurement at MV is even more difficult because the shape & size of the valve changes during the cardiac cycle
➧ The AV is the third option for Doppler assessment which can be performed using transgastric or deep transgastric views. In the absence of aortic stenosis, this method is the most accurate for CO measurements.
Disadvantages:
➧ TEE cannot be tolerated by an awake patient as a continuous CO monitor.
➧ Esophageal injury by the probe.
➧ Mediastinitis.
III. Thoracic Electrical Bioimpedance:
Principle:
➧ Changes in thoracic volume cause changes in thoracic resistance (bioimpedance) to “low amplitude, high frequency” currents. If thoracic changes in bioimpedance are measured following ventricular depolarization, SV can be continuously determined.
➧ Increasing fluid in the chest results in less electrical bioimpedance.
➧ This noninvasive technique requires 6-electrodes to inject microcurrents & to sense bioimpedance on both sides of the chest.
➧ Mathematical assumptions and correlations are then made to calculate CO from changes in bioimpedance.
Advantage:
➧ Simple, quick, non-invasive with minimal pt. risk.
Limitations:
➧ The accuracy is questionable in several groups of pt., e.g.; those with AV disease, previous heart surgery, or acute changes in thoracic sympathetic nervous function (e.g., those undergoing spinal a.).
Disadvantages:
➧ Electrode susceptibility to electrical interference.
➧ Electrode placement is an important source of error.
➧ Measurements influenced by intrathoracic fluid shifts and changes in Hct.
IV. Thoracic Bioreactance:
Principle:
➧ Because of the limitations of bioimpedance devices, newer methods of processing the impedance signal have been developed. The most promising technology to reach the marketplace is the NICOM device (Cheetah Medical, Portland, OR), which measures the bioreactance or the phase shift in voltage across the thorax.
➧ The human thorax can be described as an electric circuit with a Resistor (R) and a capacitor (C), which together create the thoracic impedance (Zo).
➧ The values of R and C determine the two components of impedance, which are:
(1) Amplitude (a), the magnitude of the impedance (measured in ohms)
(2) Phase (phi), the direction of the impedance (measured in degrees)
➧ The pulsatile ejection of blood from the heart modifies the value of R and the value of C, leading to instantaneous changes in the amplitude and the phase of Zo. Phase shifts can occur only because of pulsatile flow.
➧ The majority of thoracic pulsatile flow comes from the aorta. Therefore, the NICOM signal is correlated almost totally with the aortic flow.
➧ Furthermore, because the underlying level of thoracic fluid is relatively static, neither the underlying levels of thoracic fluids nor their changes induce any phase shifts and do not contribute to the NICOM signal.
➧ The NICOM monitor contains a highly sensitive phase detector that continuously captures thoracic phase shifts, which together result in the NICOM signal.
➧ NICOM is totally non-invasive. This system consists of a high-frequency (75 kHz) Sine wave generator and 4-dual electrode “stickers” that are used to establish electric contact with the body.
➧ Each sticker has two electrodes, one electrode is used by the high-frequency current generator to inject the high-frequency sine wave into the body, whereas the other electrode is used by the voltage input amplifier.
➧ Two stickers are placed on the right side of the body, and two stickers are placed on the left side of the body. The stickers on a given side of the body are paired, so the currents are passed between the outer electrodes of the pair, and voltages are recorded from between the inner electrodes.
➧ Thus, a non-invasive CO measurement signal is determined separately from each side of the body, and the final noninvasive CO measurement signal is obtained by averaging these two signals.
➧ The system’s signal processing unit determines the relative phase shift (∆ɸ) between the input and output signals. The peak rate of change of ɸ (dɸ/dtmax) is proportional to the peak aortic flow during each beat.
➧ The SV is calculated from the following formula: SV = C × VET × dɸ/dtmax, where C is a constant of proportionality and Ventricular Ejection Time (VET) is determined from the NICOM and electrocardiographic signals.
Advantage:
➧ Totally non-invasive.
➧ Unlike bioimpedance, bioreactance-based CO measurements do not use the static impedance (Zo) and do not depend on the distance between the electrodes for the calculations of SV, both factors that reduce the reliability of the result.
➧ NICOM averages the signal over 1 minute, therefore allowing “accurate” determination of CO in patients with atrial and ventricular arrhythmias.
➧ NICOM assessment of the CO can be performed in ventilated and non-ventilated patients alike.
➧ It is very easy to set up with a high degree of acceptability by nursing staff.
➧ NICOM assessment of the CO can be performed in the emergency room, intensive care unit, and operating room.
Limitations & Disadvantages:
➧ Electrocautery interferes with the NICOM signal. However, as long as the device receives a single for at least 20 sec. within a minute, the CO can be determined. When electrocautery is on for more than 40 sec. in a given minute, the CO for that minute is not displayed.
V. Lithium Dilution CO (LiDCO):
Principle:
➧ Depends on the “indicator dilution technique” which is minimally invasive, requiring only venous (central or peripheral) & arterial lines.
➧ The indicator is isotonic lithium chloride (LiCl) which is injected as a very small bolus (0.3 mmol) via the venous line. LiCl is not normally present in the plasma & not metabolized, and is excreted almost entirely in the urine.
➧ LiCl sensitive sensor, attached to the peripheral arterial line, detects the concentration of LiCl ions in the arterial blood.
➧ The LiCl indicator dilution “wash-out” curve provides an accurate absolute CO value.
Advantage:
➧ Simple and Minimally invasive,
➧ As accurate as, or more accurate than bolus thermodilution.
➧ Safe and does not elicit any hemodynamic changes that are sometimes seen with injections of cold saline.
Limitations & Disadvantages:
➧ The clinical margin of safety: Although the amount of LiCl injected is 100 lower than the lowest clinical doses of ‘lithium-treated patients’, it is recommended to administer not more than 10-20 boluses of lithium.
➧ Side-effects of multiple injections over a short time need to be investigated.
VI. Pulse Pressure Analysis Techniques: (Pulse Contour Analysis Devices)
Principle:
➧ Utilize the arterial pressure tracing curve to estimate the CO and other dynamic parameters; [SV, Systemic Vascular Resistance (SVR), and Blood Pressure (BP)].
➧ It measures the area of the systolic portion of the arterial pressure trace from end-diastole to the end of ventricular ejection, together with an individual calibration factor to account for individual vascular compliance.
➧ Some devices use thermo- or Li-dilution for calibration for subsequent measurement.
➧ Some devices (“FloTrac”; Edwards Life Sciences) do not require calibration with another measure and rely upon statistical analysis and algorithm.
Advantage:
➧ Offers ‘beat-to-beat’ Continuous, non-invasive CO measurement.
➧ Reliable, accurate, precise, and comparable to PA-thermodilution.
➧ Frequent recalibration or even no-calibration (“FloTrac”) is not required.
Limitations & Disadvantages:
➧ Cost.
Followers
Featured Post
Popular Posts
-
Airway Blocks 1-Superior laryngeal n. block: Block of the superior laryngeal nerve can provide anesthesia of the larynx from the epiglottis ...
-
Awareness during Anesthesia Groups at risk for awareness: Awareness is due to too light plane of anesthesia. It is more likely where mus...
-
Accidental Total Spinal Anesthesia Definition: ➧ A syndrome of the central neurological blockade. ➧ It occurs when a vol...
-
Anesthetic Considerations for Patients with Liver Disease Preoperative: 1. Assess the Degree of hepatic impairment, Severity, and Hepati...
-
Failed Spinal Anesthesia Introduction: ➧ Spinal (intrathecal) anesthesia is one of the most reliable regional block methods: the needle...
-
Laryngeal Mask Airway (LMA) 1-The LMA-Classic™: -It is a reusable LMA™ airway for general anesthesia. -The LMA-Classic™ is available in...
-
IV Fluids A) Crystalloid solutions (< 30 000 Dalton): 1-Hypotonic solutions: - D 5 W, ½ NS, D5 ¼ NS, D5 ½ NS. 2-Isotonic solutio...
-
Dexmedetomidine Mechanism of Action: -It is an imidazole derivative and is a specific alpha-2 adrenoceptor agonist that acts via post-sy...
-
Methods to stabilize ETT intracuff pressure -The use of N₂O, which is well-known to diffuse into ETT cuffs, and the lack of frequent cont...
-
Fat Embolism Syndrome (FES) ➧ Fat embolism can be difficult to diagnose. It most often follows a closed fracture of a long bone but there...