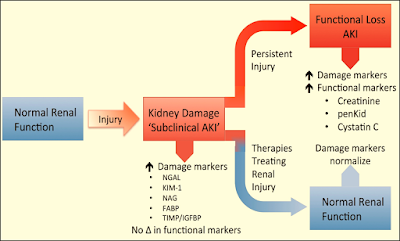
Acute Kidney Injury Biomarkers
I) Functional markers
1-Serum Creatinine (SCr):
➧ It is a degradation product of muscle cells and represents a surrogate for the efficiency of glomerular filtration.
➧ It has poor predictive accuracy for renal injury, particularly, in the early stages of AKI.
➧ In the case of critical illness, SCr concentrations are subject to large fluctuations due to a patient’s induced dilutional volume status, the catabolic effects of critical illness, the likelihood of concentration decreases in septic conditions, and the increased tubular excretion with diminishing the renal function.
➧ Furthermore, after an injurious event, the rise in SCr is slow.
➧ Therefore, detection of the earliest evidence of AKI necessitates the use of other plasma or urinary biomarkers.
2-Plasma/Serum Cystatin C (CyC):
➧ It is a 13-kDa, non-glycosylated, cysteine protease inhibitor produced by all nucleated cells at a constant rate.
➧ In healthy subjects, plasma CyC (pCyC) is excreted through glomerular filtration and metabolized completely by the proximal tubules. There is no evident tubular secretion (not detectable in urine in healthy subjects).
➧ Several studies claim the superiority of pCyC against SCr to detect minor reductions in glomerular filtration rate (GFR).
➧ It is detected in plasma and urine 12-24 h. post-renal injury.
Confounding factors: older age, Gender, Weight, Height, Systemic inflammation, High levels of C-reactive protein, Malignancy, Thyroid disorders, immunosuppressive therapy, Glucocorticoid deficiency or excess, and Smoking.
3-Fractional Excretion of sodium (FENa):
➧ (FENa) measures the percent of filtered sodium that is excreted in the urine.
➧ This calculation is widely used to help differentiate prerenal disease (decreased renal perfusion) from acute tubular necrosis (ATN) as the cause of AKI.
➧ In pre-renal azotemia, the proximal tubules reabsorb filtered sodium resulting in a very low urine sodium concentration (<20 mmol/L) and FENa is <1%.
➧ In intrinsic AKI the urine sodium concentration is >40 mmol/L and FENa is >1%.
4- Proenkephalin A (Penkid):
➧ Penkid is a 5-kDa, stable breakdown product of enkephalins.
➧ It accumulates in the blood in settings of reduced GFR.
➧ It is associated with AKI and mortality in patients with sepsis and heart failure.
II) Low-molecular-weight proteins
1-Urine Cystatin C (uCyC):
➧ The urinary excretion of CyC (uCyC) specifically reflects tubular damage because systemically produced cystatin C is normally not found in urine.
2-Urine α1/β2 microglobulin:
III) Up-regulated proteins
1-Kidney Injury Molecule-1 (KIM-1) (Cytoprotection):
➧ It is a type I transmembrane glycoprotein with a cleavable ectodomain (90-kDa).
➧ It is localized in the apical membrane of dilated tubules in an acute and chronic injury.
➧ It is produced by proximal tubular cells after ischemic or nephrotoxic injury; no systemic source.
➧ KIM-1 plays a role in regeneration processes after epithelial injury and in the removal of dead cells in the tubular lumen through phagocytosis.
➧ A reduction in proteinuria with renin-angiotensin-aldosterone blockade is accompanied by a reduction in urinary KIM-1 excretion.
➧ It is detected in urine 12-24 h. after renal injury
Confounding factors: Renal cell carcinoma, Chronic proteinuria, Chronic kidney disease, Sickle cell nephropathy.
2-Neutrophil Gelatinase-Associated Lipocalin (NGAL) (also known as oncogene 24p3) (Iron binding):
➧ It is a 25-kDa glycoprotein produced by epithelial tissues throughout the body.
➧ It is a small protein linked to neutrophil gelatinase in specific leukocyte granules.
➧ It is also expressed in a variety of epithelial tissues associated with anti-microbial defense.
➧ NGAL’s composite molecule binds ferric siderophores, induces epithelial growth, has protective effects in ischemia, and is up-regulated by systemic bacterial infections.
➧ Plasma NGAL is excreted via glomerular filtration and undergoes complete reabsorption in healthy tubular cells. It is also produced in distal tubular segments.
➧ It is detected in plasma and urine 2-4 h. after AKI.
Confounding factors: Malignancy, Chronic kidney disease, Pancreatitis, COPD, Endometrial hyperplasia.
3-Liver Fatty Acid Binding Protein (L-FABP):
➧ They are small (15-kDa) cytoplasmic proteins (intracellular lipid chaperones) produced in tissues with active fatty acid metabolism (liver, intestine, pancreas, lung, nervous system, stomach, and proximal tubular cells).
➧ Their primary function is the facilitation of long-chain fatty acid transport, the regulation of gene expression, and the reduction of oxidative stress.
➧ Urinary (L-FABP) is undetectable in healthy control urine, which is explained by efficient proximal tubular internalization via megalin-mediated endocytosis.
➧ Under ischemic conditions, tubular L-FABP gene expression is induced. L-FABP is freely filtered in glomeruli and reabsorbed in proximal tubular cells; increasing urinary excretion after tubular cell damage.
➧ In renal disease, the proximal tubular re-absorption of L-FABP is reduced.
➧ Detected in plasma and urine 1 h. after ischemic tubular injury.
Confounding factors: Chronic kidney disease, Polycystic kidney disease, Liver disease, Sepsis.
4-Interleukin-18 (IL-18):
➧ It is 18-kDa pro-inflammatory cytokine, released from proximal tubular cells following injury.
➧ Detected in plasma and urine 6-24 h. after renal injury
Confounding factors: Inflammation, Heart failure, Sepsis.
5-Tissue Inhibitor of Metallo-Proteinases-2 (TIMP-2):
6-Insulin-like Growth Factor Binding Protein-7 (IGFBP-7):
➧ They are cell cycle arrest proteins that have been suggested as early indicators of AKI.
➧ In particular, urinary (TIMP-2) and (IGFBP-7) are biomarkers of the G1 renal tubular cell cycle arrest at the early phase of AKI.
➧ The product of the urinary concentrations of TIMP-2 and IGFBP-7 (urinary [TIMP-2] × [IGFBP-7]) is a promising biomarker for the early prediction of AKI.
IV) Tubular enzymes
1-Alpha-Glutathione-s-Transferase (α-GST):
2-Pi-Glutathione-s-Transferase (π-GST):
➧ (α-GST) and (π-GST) are 47-to 51-kDa cytoplasmic enzymes.
➧ They are both members of a multigene family of detoxification enzymes present in many organs including the kidney.
➧ Distribution across the entire nephron of structurally and functionally distinct isoforms has been demonstrated.
➧ In urine, these enzymes are normally not present.
➧ After the injury, α-GST is primarily detected in the proximal cells, whereas π-GST is observed in the distal parts.
➧ They are detected in urine 12 h. after AKI.
3-Gamma-Glutamyl Transpeptidase (GGT):
4-Alkaline Phosphatase (AP):
5-Alanine Amino-Peptidase (AAP):
➧ They are tubular brush border enzymes.
➧ They are released into the urine when there has been significant damage to the brush border membrane with loss of the microvillus structures.
6-N-Acetyl-β-D-Glucosaminidase (NAG):
➧ N-Acetyl-β-D-Glucosaminidase (NAG) is a lysosomal enzyme (>130-kDa) that is localized in the renal tubules.
➧ It precludes glomerular filtration (due to large MW), implying that urinary elevations have a tubular origin.
➧ Increased activity suggests injury to its cells but may also reflect increased lysosomal activity without cell disruption.
➧ NAG catalyzes the hydrolysis of terminal glucose residues in glycoproteins.
➧ It is detected in plasma and urine 12 h. after AKI
Confounding factors: Diabetic nephropathy.
V) Others
1-Retinol Binding Protein (RBP):
➧ It is 21-kDa single-chain glycoprotein; a specific carrier for retinol in the blood (delivers retinol from the liver to peripheral tissues).
➧ It is totally filtered by the glomeruli and reabsorbed but not secreted by proximal tubules; a minor decrease in tubular function leads to the excretion of RBP in urine.
➧ It is detected in plasma and urine
Confounding factors: Type II DM, Obesity, Acute critical illness.
2-Hepcidin:
➧ It is a 2.78-kDa peptide hormone predominantly produced in hepatocytes; some production in the kidney, heart, and brain.
➧ It is freely filtered with significant tubular uptake and catabolism (fractional excretion 2%).
➧ It is detected in plasma and urine after AKI.
Confounding factors: Systemic inflammation.
3-Hepatocyte Growth Factor (HGF):
➧ It is overexpressed after AKI.
➧ It is a marker linked to renal tubular epithelial cell regeneration.
4-Netrin-1:
➧ It is a laminin-related molecule, minimally expressed in proximal tubular epithelial cells of normal kidneys.
➧ It is highly expressed in injured proximal tubules.
➧ It is detected in urine after AKI.
5-Monocyte Chemo-attractant Peptide-1 (MCP-1):
➧ It is a peptide expressed in renal mesangial cells and podocytes.
➧ It is detected in urine after AKI.
Confounding factors: Variety of primary renal diseases.
6-Calprotectin:
➧ The calcium-binding complex of two proteins of the S100 group (S100A8/ S100A9).
➧ Derived from neutrophils and monocytes.
➧ Acts as an activator of the innate immune system.
➧ It is a measure of local inflammatory activity. It is detected in urine in intrinsic AKI.
Confounding factors: Inflammatory bowel disease, Urinary tract infection, Probably CKD.
7-MicroRNA:
➧ MicroRNAs are short, non-protein-coding RNA molecules between 19 and 25 nucleotides in length.
➧ They are epigenetic regulators of gene expression at the post-transcriptional level in response to kidney injury through messenger RNA (mRNA) signal repression.
➧ As key regulators of homeostasis, their dysregulation underlies several morbidities including kidney disease.
➧ MicroRNAs are used as diagnostic and prognostic biomarkers in AKI.
8- Chitinase-3-like protein 1 (CHI3L1) (YKL-40, HC-gp39):
➧ YKL-40 is a 40-kDa heparin- and chitin-binding glycoprotein also known as Human Cartilage glycoprotein 39 (HC-gp39), 38-kDa heparin-binding glycoprotein or chitinase-3-like protein 1 (CHI3L1).
➧ The abbreviation YKL-40 is based on the one-letter code for the first three N-terminal amino acids, tyrosine (Y), lysine (K), and leucine (L), and the apparent molecular weight of YKL-40.
➧ It plays an important role in AKI and repair.